Detection of infectious agents in water from fish farms will give an indication of whether disease is developing. The method is sensitive and can be performed without handling or killing fish.

By taking a water sample from the tank or cage, it is possible to detect pathogenic organisms at an early stage. Necessary measures can then be taken before infections have become established in a large number of individuals in the fish group or spread to other parts of the facility.
Why test the water?
PCR testing of individual fish is unlikely to detect an incipient infection if the number of infected fish is low. Infected fish will excrete the disease-causing organism into the water, which in turn can infect more fish.
To detect an early-stage infection using traditional methods, a large number of fish must be removed and analysed from the group. By testing the water, we can achieve the following:
- Good fish welfare
The test method promotes fish welfare as water sampling does not involve handling or killing the fish. - Enables infection mitigation measures
Early detection of pathogenic viruses (e.g. ISAV, SAV, IPNV), bacteria (e.g. Pasteurella, Moritella) or parasites (e.g. AGD) enables the implementation of infection control measures (treatment, isolation, destruction) of fish in tanks and cages. - Risk assessment before handling fish
Regular water sampling (e.g. once a month) can be used to monitor the viral, bacterial and parasitic status of the water. Prior to stressful operations and when there is a risk of spreading infection, it can be particularly useful to know the amount of infectious agents that can cause illness after handling. - Cost-effectiveness
Sampling individual fish is a labour- and cost-intensive process that in some cases can be replaced with water samples. This will save both labour and costs as it requires fewer PCR analyses.
Experiences from facilities that perform water filtration
The filtration methodology has been field-tested since 2023. We have regularly analysed water samples from populations with low infection prevalence (<5%) and low viral loads (high Ct values). Experience data and customer feedback are very favourable. The results show that for early detection of viruses in such populations, one water sample can replace over 100 individual fish samples.
The water filtration method has also been tested in parallel with swab samples of gill and water samples from the same tank in land-based facilities. Water filtration was judged to be better as it provided higher detection of agents and was less labour-intensive and cheaper compared to sampling individual fish.
“We’ve been using water filtration as an alternative to sampling individual fish for over a year at one of our sites. This method saves both fish, labour and costs, so we will now expand its use to more locations.”
Torkjel Bruheim, Fish Health Manager at AquaGen
The test method is validated for ISAV in seawater
We have analysed water samples with different concentrations of ISAV against a sample with a known viral concentration of ISAV in seawater. The results showed that the filtration method captured between 70-90% of the viral material in the water samples with high Ct values of 29 or more. This shows that the sensitivity of the method is high and is adapted to the concentrations found in the early phase of the establishment of ISAV infections in a seawater facility.
Procedure for water sampling and filtration
The water sample must be filtered through a special filter that captures any infectious agents. Blue Analytics can assist in obtaining and installing water filtration equipment, and supplies the necessary sampling equipment. Alternatively, the water sample can be sent to Blue Analytics, which has such equipment.


Examination of pathogenic bacteria is suitable for both filter and water sample submissions. However, for the detection of viruses and parasites, we recommend that water samples are filtered on site due to the low stability of viral material in water and parasites that can attach to the inside of the water bottle. At least one litre of water should be filtered in order to achieve a high sensitivity of the analysis and have a greater chance of capturing the agent in the water mass (Figure 1).
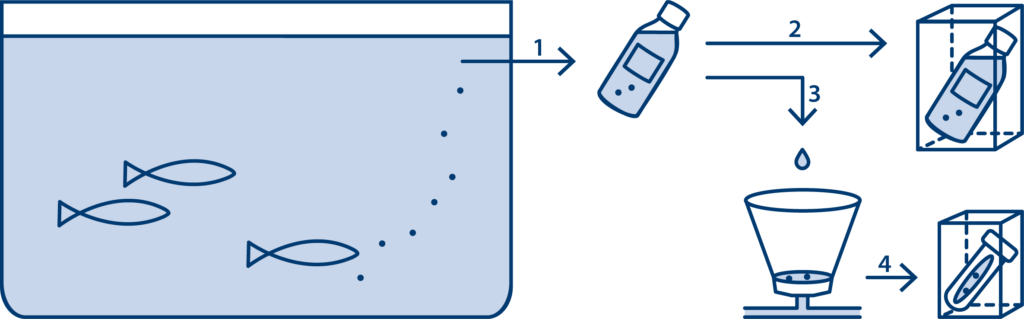
Figure 1: Procedure for water sampling and water filtration for further detection of infection, with or without access to water filtration equipment at the facility.
Without access to water filtration equipment:
1: Take a water sample at the water outlet, 2: Send the water bottle to Blue Analytics for PCR analysis.
With access to water filtration equipment:
1: Take water sample at the water outlet, 3: Pour the water into the funnel with filter, 4: Send the filter to Blue Analytics for PCR analysis.
Make a needs assessment of the number of water samples per tank or cage and sampling frequency based on the fish population and the expected prevalence of target organisms. Feel free to contact the head of lab analyses and operations, Dag Knappskog, during the planning phase of the sampling (dag.knappskog@blueanalytics.no, mobile: 414 34 424).
Here are links to detailed descriptions of the procedure and ordering of PCR analysis (under Diagnostics ) and ordering of sampling kits for taking water samples
